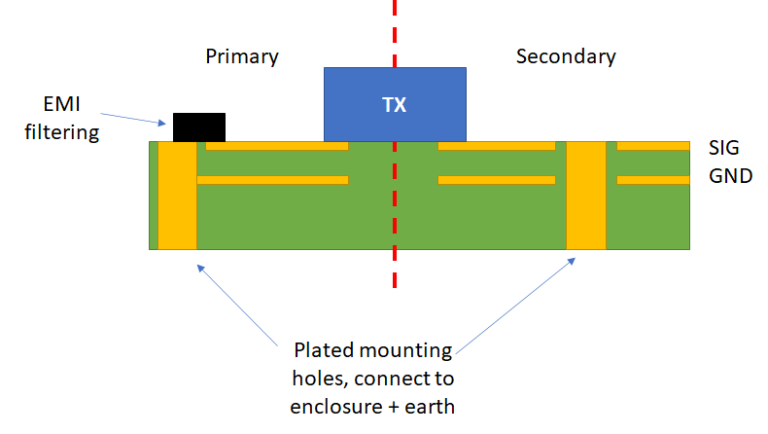
Medical PCB Manufacturer for Advanced Healthcare Technology
Key Takeaways
When selecting a PCB manufacturing partner for medical devices, you need a provider that balances precision, compliance, and scalability. Leading PCB manufacturing companies prioritize certifications like IPC-6012 and UL 94 V-0, ensuring designs meet rigorous medical-grade standards for safety and performance. This requires not only advanced engineering expertise but also a deep understanding of how PCB manufacturing cost impacts budgets without compromising on traceability or material quality.
For healthcare applications, prototyping and volume production must align with strict regulatory frameworks. A reliable PCB manufacturing business will offer end-to-end support—from rapid prototyping for new product introduction (NPI) to scalable solutions for high-volume demands. Lifecycle engineering services further ensure long-term reliability, critical for devices like patient monitors or implantable technologies.
Transitioning between development phases demands seamless coordination. Whether you’re optimizing PCB manufacturing processes for biocompatibility or validating designs through accelerated lifecycle testing, your partner should integrate quality checks at every stage. Learn how trusted certifications strengthen medical device innovation. By prioritizing these factors, you mitigate risks while accelerating time-to-market for life-critical technologies.

High-Reliability Medical PCB Manufacturing
When designing medical devices, PCB manufacturing must prioritize uncompromising reliability to meet stringent healthcare demands. Leading PCB manufacturing companies leverage advanced processes to ensure traceability, defect-free assembly, and compliance with IPC-6012 (for rigid boards) and UL 94 V-0 (flammability resistance). These standards are critical for applications like patient monitors or implantable devices, where failure is not an option.
Balancing PCB manufacturing cost with performance requires expertise in material selection and design optimization. For instance, high-temperature laminates or controlled impedance layouts may increase initial expenses but reduce long-term risks. Below is a comparison of key considerations:
| Factor | Impact on Reliability |
|---|---|
| Material Grade | Higher thermal/mechanical stability |
| Trace Precision | Reduced signal loss/interference |
| Testing Protocols | Early fault detection |
In the PCB manufacturing business, scalability is equally vital. Partnering with firms offering end-to-end lifecycle support ensures seamless transitions from prototyping to mass production. This approach minimizes redesigns, accelerates time-to-market, and maintains consistency across batches—critical for FDA-approved devices. By aligning your requirements with certified PCB manufacturing partners, you safeguard both patient safety and operational efficiency.

Precision PCB Design for Healthcare Devices
When developing medical devices, precision in PCB manufacturing isn’t optional—it’s a necessity. Every millimeter of trace spacing, component placement, and impedance control directly impacts the reliability of diagnostic tools, implantable devices, or monitoring systems. Leading PCB manufacturing companies leverage advanced design software to simulate thermal performance, signal integrity, and EMI shielding before prototyping begins, reducing risks in critical healthcare applications.
Tip: Always prioritize design-for-manufacturability (DFM) reviews early in the process—this minimizes redesign cycles and keeps PCB manufacturing cost predictable, even for complex multilayer boards.
Your choice of partner matters: specialized firms in the PCB manufacturing business understand the unique tolerances required for medical-grade boards. For example, rigid-flex designs for wearable monitors demand precise layer alignment, while high-density interconnects (HDIs) in imaging systems require microvia drilling accuracies under 50µm. Compliance with standards like IPC-6012 Class 3 ensures defect-free performance in life-critical environments.
By integrating lifecycle engineering support from the design phase, you avoid costly mid-production changes. This approach also streamlines scalability, allowing seamless transitions from low-volume prototypes to full-scale production without sacrificing quality—a key advantage when timelines for FDA approvals or CE certifications are tight.

Prototyping to Volume Production Solutions
When developing medical devices, the journey from concept validation to full-scale deployment demands a seamless transition between prototyping and volume PCB manufacturing. Leading PCB manufacturing companies specializing in healthcare technologies understand that initial prototypes require rigorous testing to meet biocompatibility and signal integrity standards. As you scale from low-volume batches to mass production, balancing PCB manufacturing cost with uncompromised quality becomes critical. Advanced manufacturers leverage automated assembly lines and DFM (Design for Manufacturability) principles to optimize yield rates while maintaining traceability for regulatory compliance.
For medical applications, the shift from rapid prototyping to high-volume output hinges on selecting partners with proven expertise in PCB manufacturing business dynamics. Look for suppliers offering flexible scaling options—whether you need 50 units for clinical trials or 50,000 for global distribution. By aligning with a manufacturer that integrates cleanroom environments and ISO 13485-certified processes, you ensure consistency across all production phases. This approach minimizes risks of design flaws or delays, enabling faster time-to-market for life-saving technologies.

Lifecycle Engineering Support for Medtech
When developing medical devices, you need more than just a one-time production partner—you require PCB manufacturing companies that offer comprehensive lifecycle engineering support. This means collaborating with experts who understand how PCB manufacturing cost and quality evolve across a product’s lifespan, from initial prototyping to end-of-life transitions. By integrating PCB manufacturing expertise early in your design phase, teams can optimize layouts for manufacturability, mitigate risks like component obsolescence, and ensure compliance with IPC-6012 and UL 94 V-0 standards—critical for medical-grade reliability.
Leading providers in the PCB manufacturing business extend support beyond assembly, offering failure analysis, redesigns for regulatory updates, and supply chain continuity planning. For instance, if your device requires a material shift due to new biocompatibility guidelines, engineers can recalibrate designs without disrupting production timelines. This approach not only reduces long-term operational costs but also ensures your medtech solutions remain scalable and compliant in dynamic healthcare markets. By prioritizing lifecycle partnerships, you align with partners invested in your product’s success—not just its initial rollout.
Quality Testing in Medical PCB Assembly
When selecting PCB manufacturing companies for medical devices, rigorous quality testing isn’t optional—it’s a non-negotiable requirement. Medical-grade PCBs must perform flawlessly in life-critical applications, which is why advanced testing protocols are embedded at every stage of production. From automated optical inspection (AOI) to X-ray verification of high-density interconnects, each layer undergoes scrutiny to detect microfractures, solder voids, or trace misalignments. Functional testing under simulated operational stress—thermal cycling, vibration, and humidity exposure—ensures boards withstand real-world healthcare environments.
For cost-sensitive projects, balancing PCB manufacturing cost with reliability is key. Reputable providers use statistical process control (SPC) to minimize defects early, avoiding expensive rework. Traceability systems document every component’s origin, crucial for FDA audits and post-market surveillance. While stringent testing adds upfront time, it reduces long-term risks like field failures or recalls—a critical factor in the PCB manufacturing business where compliance gaps can derail market approvals.
Transitioning from prototyping to volume production? Cross-functional test jigs validate design-for-manufacturability (DFM) improvements, ensuring scalability without compromising IPC-6012 Class 3 standards. By prioritizing zero-defect workflows, manufacturers align with your need for devices that meet both technical specifications and patient safety imperatives.
IPC-6012 & UL 94 V-0 Compliance Standards
When partnering with PCB manufacturing companies for medical devices, compliance with IPC-6012 and UL 94 V-0 standards ensures reliability in critical healthcare applications. The IPC-6012 specification governs the performance requirements for rigid printed boards, emphasizing durability under thermal cycling, mechanical stress, and prolonged operation—non-negotiable factors for medical PCB manufacturing. Meanwhile, UL 94 V-0 certification guarantees flame-retardant materials, minimizing fire risks in oxygen-rich medical environments.
Balancing PCB manufacturing cost with adherence to these standards requires expertise. Reputable providers integrate compliance into every phase, from material selection to final testing, avoiding costly redesigns or recalls. For instance, using UL-approved substrates might raise initial expenses but reduces long-term liabilities. This approach aligns with the operational priorities of a PCB manufacturing business focused on medical-grade quality, where cutting corners jeopardizes patient safety and regulatory approvals.
Your choice of manufacturer should prioritize certifications as proof of capability, not just marketing claims. Compliance ensures designs meet global regulatory benchmarks, safeguarding both device functionality and your brand’s reputation in the competitive PCB manufacturing landscape.
Global Medical PCB Manufacturing Services
When developing medical devices for international markets, partnering with PCB manufacturing companies that operate globally ensures seamless scalability and compliance with regional regulations. These providers combine localized expertise with PCB manufacturing infrastructure spanning multiple continents, enabling faster turnaround times and optimized PCB manufacturing cost through strategic material sourcing and logistics networks. By leveraging facilities in North America, Europe, and Asia, you gain access to region-specific certifications—such as CE marking or FDA-aligned quality systems—while maintaining consistency in high-reliability designs.
A robust PCB manufacturing business focused on healthcare prioritizes agility, adapting to supply chain shifts without compromising on traceability or IPC-6012 standards. For instance, regional hubs can expedite prototyping for urgent medtech innovations while coordinating volume production across sites to meet demand spikes. This distributed model also mitigates risks like component shortages, ensuring your critical medical devices remain on schedule.
Transitioning between prototyping and full-scale production becomes smoother when your partner’s global medical PCB manufacturing services include unified documentation and lifecycle management tools. This alignment reduces validation hurdles, allowing you to focus on advancing patient-centric technologies without geographic constraints.
Medical Device PCB Prototyping & NPI
When developing cutting-edge medical devices, the PCB manufacturing phase begins with rigorous prototyping and new product introduction (NPI). This stage demands precision, as even minor design flaws can delay regulatory approvals or compromise patient safety. Leading PCB manufacturing companies leverage advanced tools like impedance-controlled routing and multilayer stack-up simulations to refine designs before production.
Balancing PCB manufacturing cost with quality is critical here. Prototyping often involves iterative testing, which can escalate expenses if not managed strategically. By collaborating with partners experienced in medical-grade materials and IPC-6012 standards, you ensure compliance while optimizing budgets. For instance, design-for-manufacturability (DFM) feedback during NPI minimizes rework, accelerating time-to-market.
Transitioning from prototypes to volume production requires seamless scalability—a hallmark of trusted PCB manufacturing business models. These providers integrate automated testing and traceability systems to maintain consistency across batches, crucial for FDA-compliant devices. Whether you’re iterating a wearable monitor or a surgical robot, aligning with a partner skilled in lifecycle engineering ensures your project stays on track, from concept to clinical deployment.
Conclusion
When selecting a PCB manufacturing partner for medical devices, prioritizing expertise in high-reliability solutions is non-negotiable. Leading PCB manufacturing companies in the healthcare sector combine rigorous compliance with IPC-6012 and UL 94 V-0 standards, ensuring designs meet the stringent demands of critical medical applications. While PCB manufacturing cost remains a consideration, partnering with specialists ensures value through minimized risks, reduced rework, and lifecycle support—key factors for long-term success in the PCB manufacturing business.
Your choice should balance scalability (from rapid prototyping to volume production) with traceability and documentation, which are vital for regulatory approvals. By aligning with manufacturers that offer global services, you gain access to localized expertise without compromising on quality or turnaround times. Ultimately, the right partner doesn’t just deliver boards—they become an extension of your engineering team, safeguarding the integrity of life-saving technologies.
Frequently Asked Questions
How do medical PCB manufacturing processes ensure reliability in healthcare devices?
Medical-grade PCB manufacturing follows stringent protocols, including IPC-6012 and UL 94 V-0 certifications, to guarantee performance in critical environments. Every design undergoes rigorous testing for thermal stability and signal integrity to meet healthcare safety standards.
What factors influence PCB manufacturing cost for medical applications?
Cost depends on material selection, layer count, and compliance requirements. High-reliability substrates like polyimide and embedded trace testing often increase expenses, but partnering with specialized PCB manufacturing companies ensures cost-efficiency without compromising quality.
Why choose a PCB manufacturing business focused on medical devices?
Dedicated manufacturers offer lifecycle support, from prototyping to volume production, ensuring seamless integration with FDA-approved systems. Their expertise in medical-grade PCB manufacturing reduces design risks and accelerates time-to-market for innovations like diagnostic tools or implantable devices.
How do PCB manufacturing companies handle low-volume prototyping for medtech?
Leading providers prioritize rapid prototyping with full design validation, enabling iterative testing for compliance and functionality. This approach minimizes delays during new product introduction (NPI) phases while maintaining scalability for future production needs.
Ready to Elevate Your Medical Device Innovation?
For tailored solutions in high-reliability PCB manufacturing, please click here to connect with experts specializing in advanced healthcare technologies.